How Your Gut’s Mucus Layer Protects You from Microplastics
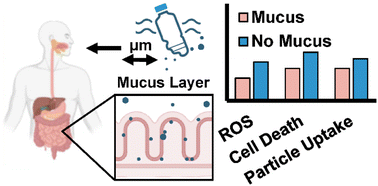
With support from The Herbert W. Hoover Foundation, researchers at Cornell University published new findings in Biomaterials Science showing that human intestinal mucus plays a critical role in blocking microplastics from entering gut tissue. The study revealed that an intact mucus barrier significantly reduces cellular uptake, inflammation, and oxidative stress caused by plastic particles ranging from 40 to 500 nm. Particle size, surface chemistry, and composition all influenced how easily plastics migrated through the barrier. These results deepen our understanding of how microplastics affect human health and highlight the importance of gut integrity in protecting against environmental pollutants. The full article is available here.
Can Ocean Plastics Change the Weather? A New Study Says Maybe
A recent study published in Environmental Science: Atmospheres revealed that nanoplastics — tiny particles from degraded ocean plastics — can disrupt fatty-acid films at the ocean’s surface, which play a key role in how gases and particles move between the ocean and the atmosphere. These changes may affect the formation and behavior of sea spray aerosols, which influence cloud formation and climate processes. The research highlights a new potential pathway for plastic pollution to impact our climate. This paper was funded in part by The Herbert W. Hoover Foundation and featured on the issue cover of Environmental Science: Atmospheres.
For more information, please revisit: https://pubs.rsc.org/en/content/articlelanding/2025/ea/d5ea00075k
Microplastics and “Negative Neuroplasticity” — Why Tiny Plastics May Threaten Brain Health
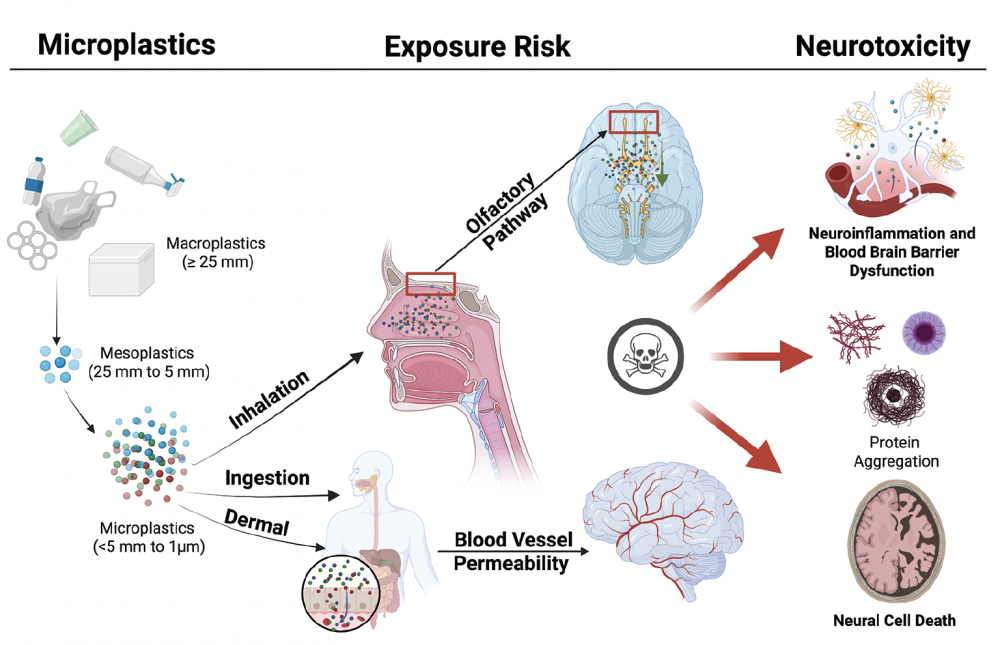
Funded by the Herbert W. Hoover Foundation, a recent review in Practical Neurology examines emerging evidence that microplastics – tiny fragments from degraded or single‑use plastics – can enter and accumulate in the human brain, potentially contributing to neurodegenerative and cerebrovascular illnesses such as dementia, Parkinson’s disease, and stroke.
The authors describe multiple pathways through which microplastics may reach the brain — including inhalation (via the olfactory nerve), ingestion (via the gut–brain axis), or direct passage through a compromised blood–brain barrier.
Once within the brain, microplastics may trigger harmful effects such as oxidative stress, neuroinflammation, disruption of neurotransmitter systems, and aggregation of disease‑associated proteins like amyloid‑β and α‑synuclein.
The article calls attention to significant research gaps — especially around dose, exposure route, and long-term impacts — and advocates reducing exposure to plastics as the most practical step for now. Read the full review here.
New Study Quantifies How Mangroves Cut Property Damage During Hurricanes
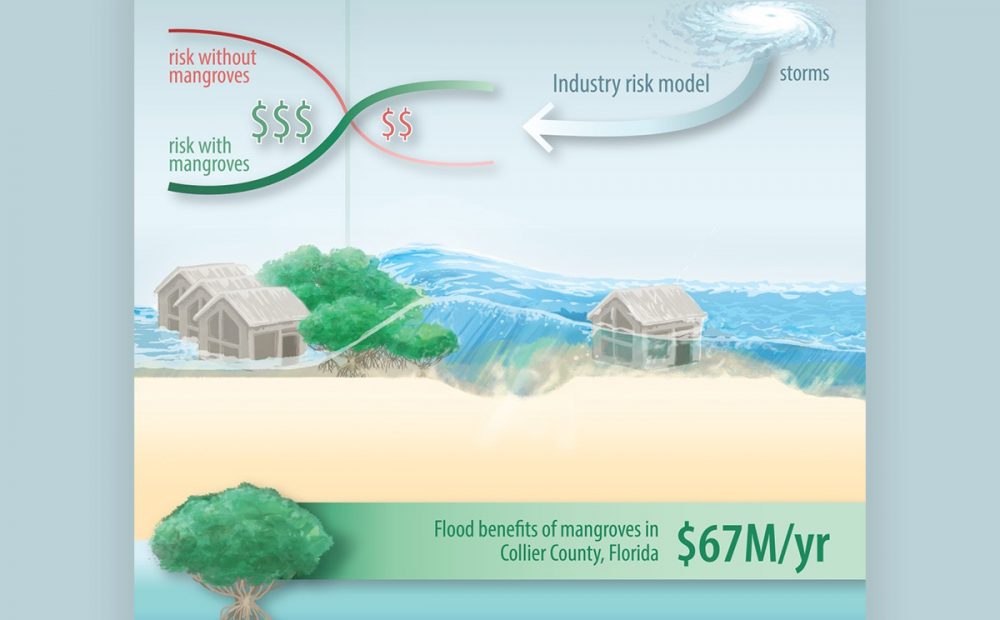
A recent collaboration between UC Santa Cruz’s Center for Coastal Climate Resilience and East Carolina University found that mangrove forests significantly mitigate storm surge damage to inland properties. Using industry-grade catastrophe risk models, the team estimated mangrove-related benefits of $725 million during Hurricane Irma and $4.1 billion during Hurricane Ian. For properties in southwest Florida’s Collier County, the annual value of mangrove-mediated flood protection was estimated at around $67 million. The research is the first to incorporate catastrophe‑modeling tools to assign monetary value to natural coastal defenses. Funding support for this work included a grant from The Herbert W. Hoover Foundation. You can access the full article by visiting this link.
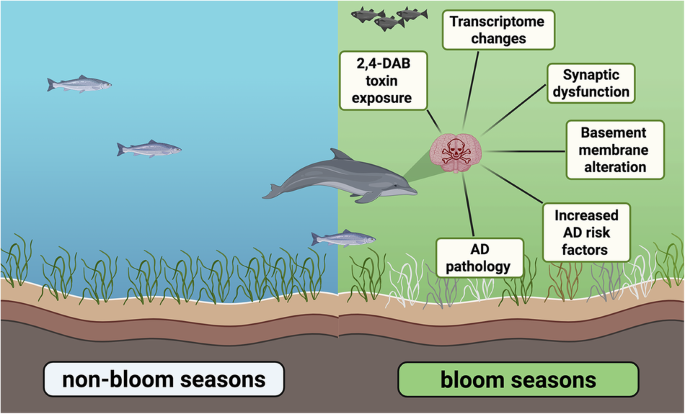
Harmful algal blooms linked to Alzheimer’s-like brain changes in Florida’s Indian River Lagoon dolphins
HWHF-supported researchers investigated how harmful algal bloom (HAB) exposure impacts brain health by studying stranded dolphins in Florida’s Indian River Lagoon. They found that levels of the neurotoxin 2,4-diaminobutyric acid (2,4-DAB) were 2,900 times higher in dolphin brains during HAB seasons than non-bloom periods. These dolphins exhibited over 500 altered genes associated with Alzheimer’s disease (AD), GABAergic synapse impairment, and structural brain changes. The findings show that repeated seasonal exposure to 2,4-DAB may accelerate AD-like pathology in marine mammals, with implications for other species as HABs intensify under climate change. To read the full article, visit this link: https://www.nature.com/articles/s42003-025-08796-0
‘Defend the Deep’ Documentary Wins Big in 2025
We are delighted to announce that the HWHF-backed documentary “Defend the Deep” has been recognized for several awards this year, including:
- Best Environmental at the Tokyo International Cinema Awards 2025
- Best Documentary Short at the Boston Indie Film Festival 2025
- Best Documentary Short at the Dallas Movie Fest in July 2025
- Marine Sciences Award at the International Ocean Film Festival 2025
- Official Selection at the International Ocean Film Festival 2025
- Official Selection at the Korea International Ocean Film Festival 2025
- Semi-Finalist at the Paris International Short Festival May 2025
- Official Selection at the Cannes Arts Film Fest 2025
- Ocean Life Documentary Award at the Nature Without Borders International Film Festival 2025
Additionally, the documentary has also received the following awards:


The award-winning documentary was directed by Richard Allen Charter and Liz Ruben.
The film can be viewed online on YouTube at the following link: https://www.youtube.com/watch?v=YaQMfOzJg_4&t=2s
Medical Science Meets Ocean Rescue: New Fellowship Bridges the Gap
How Biomedical Innovation Is Powering Marine Conservation
Crosscutting fellowship links ocean and human health
Human well-being and ocean health are closely intertwined: The marine environment plays a critical role in climate regulation and provides important resources to people—from food and medicines to nutritional supplements, coastline protection, and materials used in agriculture, cosmetics, and construction. But our oceans also face unprecedented threats, including pollution, overfishing, and climate change.
Marine conservation research is vital for finding solutions to these challenges and informing management decisions to protect ocean ecosystems. To strengthen connections between marine and biomedical sciences and foster innovative research with the potential to improve human health, The Pew Fellows Program in Marine Conservation and the Pew Scholars Program in the Biomedical Sciences have partnered with the Herbert W. Hoover Foundation on a fellowship positioned at the intersection of the two research fields.
Building bridges between disciplines
Much of the cutting-edge science being applied in marine conservation research today—including techniques such as gene editing and monitoring for environmental contaminants—has roots in the biomedical field, which is larger and typically better resourced. The new fellowship is designed to accelerate the transfer of techniques from biomedicine to marine science, with the goal of benefitting both people and the environment.
“We are thrilled to help launch this collaboration between the two Pew programs,” said Caiti Waks, program and outreach director for the Herbert W. Hoover Foundation. “Each program has a proven track record of identifying exceptional scientists and cultivating vibrant, collaborative networks within their respective communities. This crosscutting fellowship builds on those strengths by forging meaningful connections between the two programs.”
Supporting human and ocean health
Robert Richmond, a 2006 Pew marine fellow based at the University of Hawaii at Manoa, has seen firsthand the value of exchanges between marine science and biomedical research.
“Collaboration with colleagues in pharmacology has been critical to our success in advancing coral reef conservation,” Richmond explained. “By harnessing biomedical tools and approaches, we can now identify, understand, and address the sources of coral stress at sublethal levels—from chemicals found in common sunscreens to swimming pool water in runoff—and reduce them before corals lose reproductive capacity, bleach, or die.
“The crossover tools and techniques allow us to move from correlation to actual causation to diagnose and treat affected corals and evaluate the effectiveness of management interventions in real time, from days to weeks rather than months to years.”

Tools and approaches such as advanced imaging and bioinformatics, which uses computers to analyze complex biological information, also have roots in biomedicine and are now commonly applied in marine science. These techniques have enabled researchers to evaluate the biodiversity effects of conservation interventions, such as oyster reef restoration, and to better understand and manage the population dynamics of vulnerable marine species.
Taking inspiration from the sea
Phillip Cleves, selected in 2023 as the first recipient of the Pew-Hoover Fellowship in Marine and Biomedical Science, uses gene editing techniques to understand the factors that make some corals more likely to survive and recover from heat stress. He hopes to use this information to improve coral reef restoration by enabling practitioners to find wild corals with these traits so they can be propagated and protected—making reefs more resilient to climate impacts.
As a member of both Pew programs, Cleves has helped link the two robust, but often siloed, communities of scientific expertise.
“The [Pew-Hoover] fellowship presents the possibility of taking technology built for medicine and applying that technology to ecological problems, with the deep understanding that ecological health is human health,” Cleves said. “The connection between environmental and human health will only become more apparent as ecosystems degrade and pollution and populations increase, impacting our environment and contributing to human disease and suffering.”

Marine life such as corals and anemones can be useful model organisms for biomedical research, thanks to their extreme longevity and regeneration. But they remain a largely untapped resource that could help answer larger conservation questions as well as unlock new scientific insights that benefit both fields.
Accelerating progress in ocean protection
“The Herbert W. Hoover Foundation has a long history of supporting interdisciplinary work, particularly at the intersection of marine and human health,” Waks explained. “Recent HWHF-funded research identified links between harmful algal blooms in Florida and increased rates of neurodegenerative diseases in dolphins.”
“Subsequent studies in primates revealed similar patterns, demonstrating the neurotoxic effect harmful algal blooms can have on local populations,” she added. “Uncovering these connections is not only critical for protecting human health but also for inspiring public engagement. When people understand how environmental issues impact their own well-being, they’re far more likely to take action to protect both the environment and their health.”

“Ocean research has vast potential to benefit people—from drug discovery to improved environmental safety,” said Angela Bednarek, director of Pew’s scientific advancement portfolio. “The Pew Fellows Program in Marine and Biomedical Science is accelerating this type of interdisciplinary work to drive innovative solutions for human and ocean health.”
The next Pew-Hoover fellow will be announced in 2026.
Authored by Nathan Fedrizzi. Nathan Fedrizzi works on the Pew Fellows Program in Marine Conservation. To view the full article, please visit: https://www.pew.org/it/research-and-analysis/articles/2025/04/16/how-biomedical-innovation-is-powering-marine-conservation
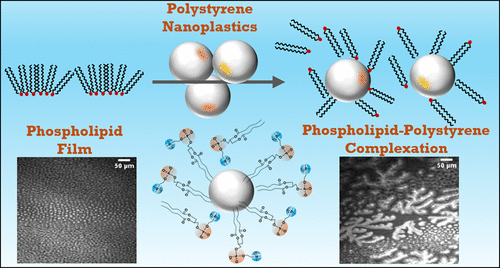
How Nanoplastics Disrupt Cellular Membrane Structure
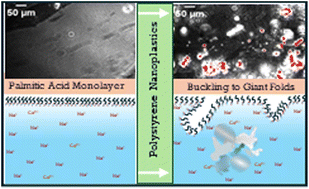
Nanoplastic-Induced Disruption of DPPC and Palmitic Acid Films: Implications for Membrane Integrity
With funding from the Herbert W. Hoover Foundation, Dr. Heather Allen of The Ohio State University published a study exploring how polystyrene nanoplastics—tiny plastic particles formed from the breakdown of larger plastics—interact with biological membrane components and potentially disrupt cellular function. The research demonstrated that nanoplastics can embed themselves into lipid films that mimic lung surfactants and cell membranes, altering their structure and potentially impairing critical biological processes like respiration, nutrient absorption, and cellular communication. These findings deepen our understanding of how nanoplastics may accumulate in living organisms and interfere with health at the molecular level. The study, conducted by Dr. Allen and her co-authors, is published in Environmental Science & Technology and can be accessed here (Allen et al., 2020).
Insights from Axolots into Human Spinal Chord Regeneration
Neuronal activation in the axolotl brain promotes tail regeneration
With support from the Herbert W. Hoover Foundation, Dr. Karen Echeverri of the Marine Biological Laboratory led a study published in npj Regenerative Medicine that uncovers a critical role for brain neurons in spinal cord regeneration in axolotls. The team’s findings reveal that neuron activation in brain regions distant from the injury site—not just in local tissue—plays a crucial, long-distance role in orchestrating regenerative repair through specific neuropeptides. This breakthrough challenges traditional assumptions that regeneration is driven only by cells near the wound and opens new avenues for exploring how the brain might influence healing processes throughout the body. The work holds potential implications for regenerative medicine, including the possibility of stimulating similar pathways in humans. The full study is available in npj Regenerative Medicine here.